APLENZIN is bioequivalent to bupropion HCl.
*This offer is not valid for patients covered by Medicare, Medicaid or any other federal or state funded healthcare program or where prohibited by law. Click here for full eligibility criteria, terms and conditions.
To help ensure you get APLENZIN as prescribed, ask your healthcare professional to write "Dispense as Written" (DAW1) on your prescription.
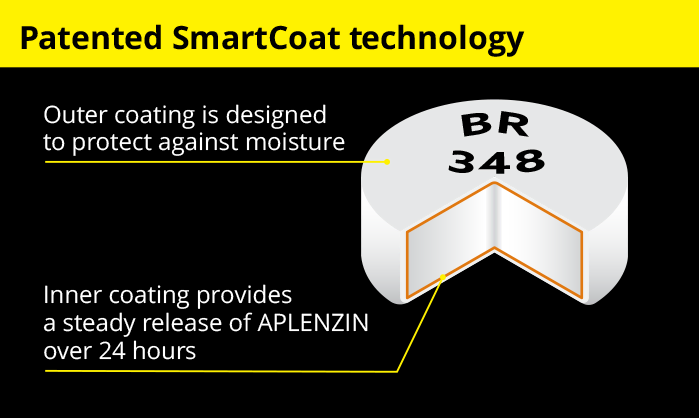
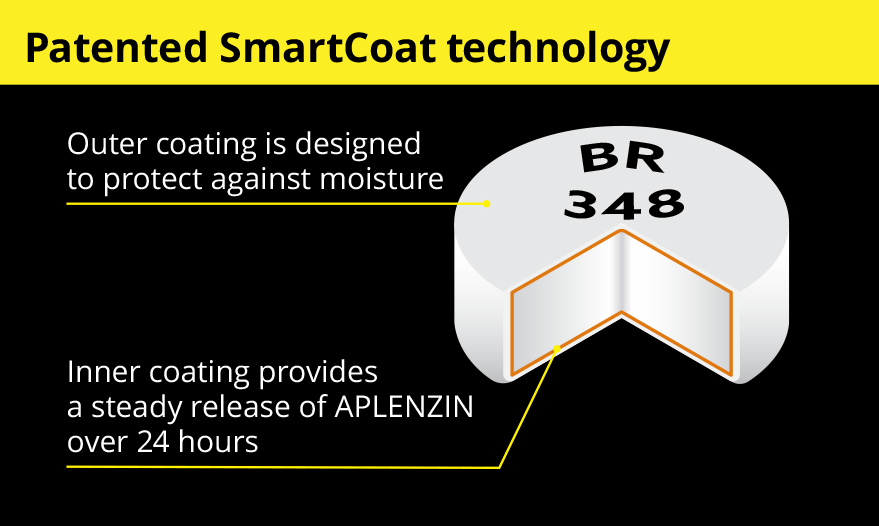
APLENZIN features a patented technology called SmartCoatTM. It allows for a steady release of each APLENZIN tablet over 24 hours. This means a consistent release of the active ingredient is made available in the body over the course of each day.
See how SmartCoat technology works
See how SmartCoat technology worksWatch the video![]()
APLENZIN® (bupropion hydrobromide) extended-release tablets is a prescription medicine used to treat adults with a certain type of depression called major depressive disorder, and for the prevention of autumn-winter seasonal depression (seasonal affective disorder).
WARNING: CHANGES IN THINKING AND BEHAVIOR, DEPRESSION, AND SUICIDAL THOUGHTS OR ACTIONS
Suicidal Thoughts or Actions and Antidepressant Drugs
Antidepressants may increase the risk of suicidal thoughts or actions in some children, teenagers, or young adults within the first few months of treatment. Depression or other serious mental illnesses are the most important causes of suicidal thoughts and actions. People who have (or have a family history of) bipolar illness or suicidal thoughts or actions may have a particularly high risk. Pay close attention to any changes, especially sudden changes in mood, behaviors, thoughts, or feelings. Call your healthcare provider right away if symptoms such as anxiety, irritability, impulsivity, trouble sleeping, aggressive behavior or suicidal thoughts are new, worse or worry you. APLENZIN has not been evaluated for use in patients under the age of 18.
APLENZIN® (bupropion hydrobromide) extended-release tablets is a prescription medicine used to treat adults with a certain type of depression called major depressive disorder, and for the prevention of autumn-winter seasonal depression (seasonal affective disorder).
WARNING: CHANGES IN THINKING AND BEHAVIOR, DEPRESSION, AND SUICIDAL THOUGHTS OR ACTIONS
Suicidal Thoughts or Actions and Antidepressant Drugs
Antidepressants may increase the risk of suicidal thoughts or actions in some children, teenagers, or young adults within the first few months of treatment. Depression or other serious mental illnesses are the most important causes of suicidal thoughts and actions. People who have (or have a family history of) bipolar illness or suicidal thoughts or actions may have a particularly high risk. Pay close attention to any changes, especially sudden changes in mood, behaviors, thoughts, or feelings. Call your healthcare provider right away if symptoms such as anxiety, irritability, impulsivity, trouble sleeping, aggressive behavior or suicidal thoughts are new, worse or worry you. APLENZIN has not been evaluated for use in patients under the age of 18.
Call your healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
Although APLENZIN is not a treatment for quitting smoking, it contains the same active ingredient (bupropion) as ZYBAN, which is used to help patients quit smoking.
Some people have had serious side effects while taking bupropion to help them quit smoking, including:
New or worse mental health problems, such as changes in behavior or thinking, aggression, hostility, agitation, depression, or suicidal thoughts or actions. Some people had these symptoms when they began taking bupropion, and others developed them after several weeks of treatment, or after stopping bupropion. These symptoms happened more often in people who had a history of mental health problems before taking bupropion than in people without a history of mental health problems.
Stop taking APLENZIN and call your healthcare provider right away if you, your family, or caregiver notice any of these symptoms. Work with your healthcare provider to decide whether you should continue to take APLENZIN. In many people, these symptoms went away after stopping APLENZIN, but in some people, symptoms continued after stopping APLENZIN. It is important for you to follow-up with your healthcare provider until your symptoms go away.
Before taking APLENZIN, tell your healthcare provider if you have ever had depression or other mental health problems. You should also tell your healthcare provider about any symptoms you had during other times you tried to quit smoking, with or without bupropion.
What Other Important Information Should I Know About APLENZIN?
Do not take APLENZIN if you:
Although APLENZIN is not a treatment for quitting smoking, it contains the same active ingredient (bupropion) as ZYBAN, which is used to help patients quit smoking. Before taking APLENZIN, tell your healthcare provider if you have ever had depression, suicidal thoughts or actions, or other mental health problems or any symptoms you had during other times you tried to quit smoking with or without bupropion. Also, tell your healthcare provider about your other medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Many medicines increase your chances of having seizures or cause other serious side effects if you take them while you are using APLENZIN.
The most common side effects of APLENZIN include: trouble sleeping, stuffy nose, dry mouth, dizziness, feeling anxious, nausea, constipation, and joint aches.
Click here to read the Medication Guide carefully before you start using APLENZIN. If you have any questions about APLENZIN, ask your healthcare provider or pharmacist.
Click here for full Prescribing Information, including Medication Guide and Boxed Warning regarding suicidal thoughts and actions.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. You may also contact Bausch Health Customer Service at 1-800-321-4576.